Solving Documentation Challenges
The Shift From PDFs To Integrated System
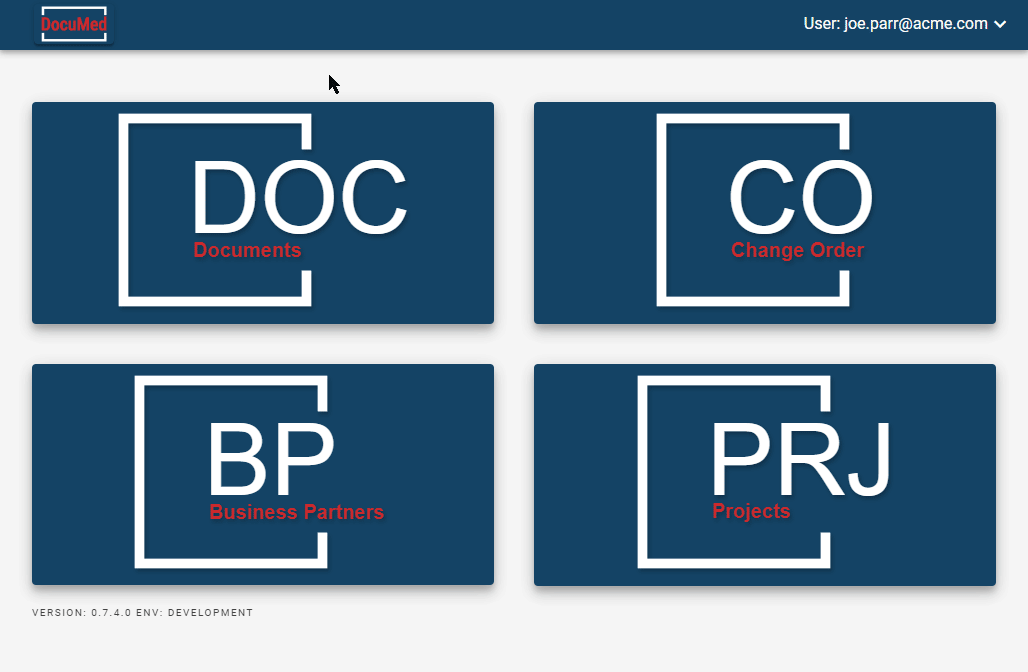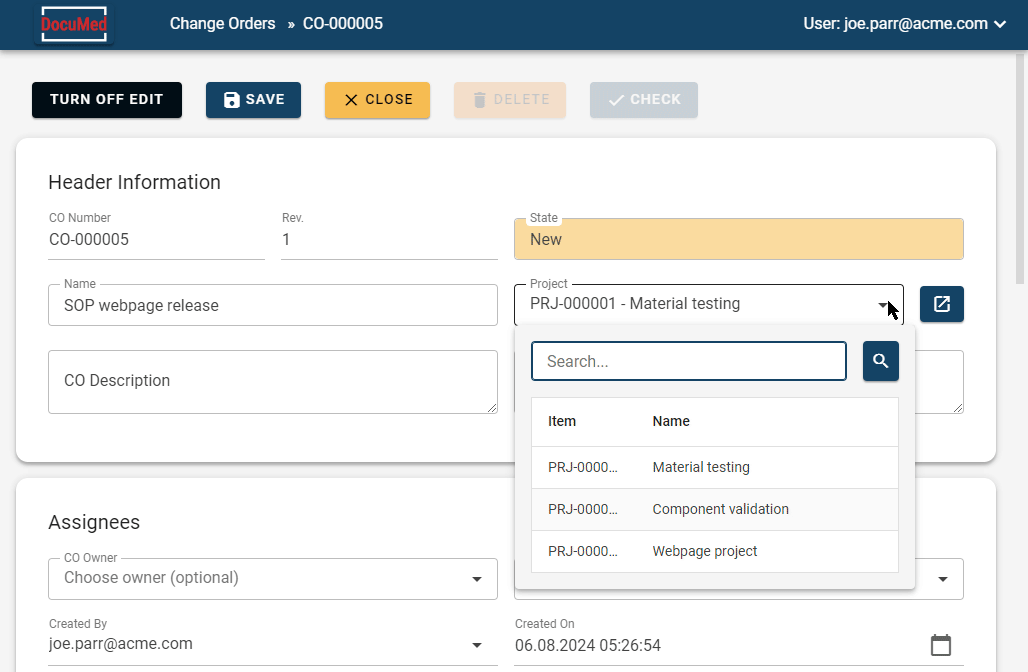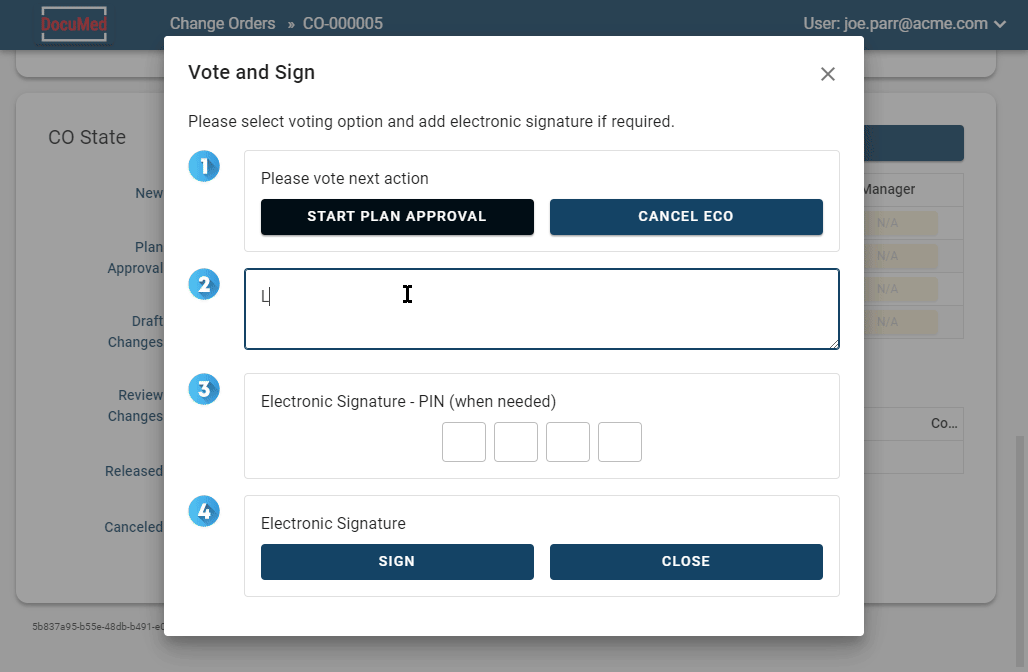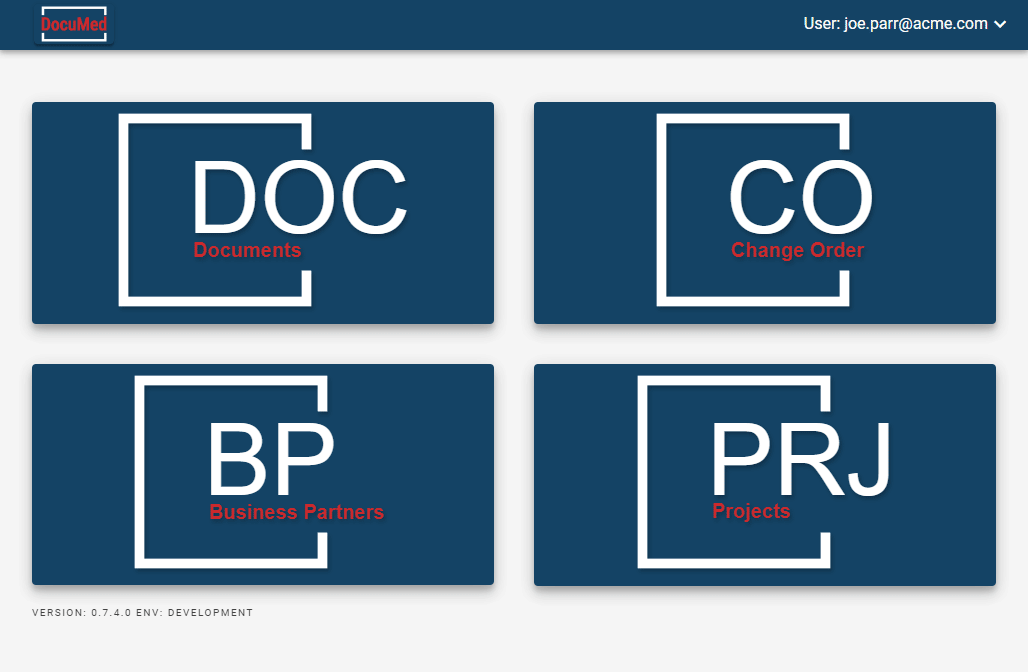
Traditional methods of handling medical device documentation often involve cumbersome and error-prone processes. This includes managing paper documents, PDFs, and using network shared folders or services like Dropbox or SharePoint.
These methods can lead to:
- Version control issues
- Lack of real-time collaboration
- Security vulnerabilities
- Time-consuming navigation through scattered documents
- Confusion in ensuring the latest version is in use
DocuMed offers a solution for managing medical device documentation, including change control and electronic signatures, making it easy for teams to collaborate effectively and securely.


